Photoelectric Effect
The ultraviolet sources used in this lab are intended for germ sterilization and emit light in the UV-C range, which can be damaging to skin or eyesight. Do not shine them on bare skin or toward anyone’s face.
Objectives
- Demonstrate the photoelectric effect by shining a light on a metal surface and showing that electrons are ejected.
- Show that the photoelectric effect depends on the frequency, not intensity, of the light.
- Calculate the work function for the metal you use.
Resources
- UV-C light source
- LED flashlight
- Aluminum can electroscope
- friction rods, silk, animal fur, etc.
Background
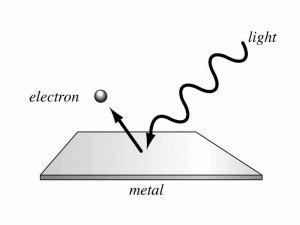 Many metals will emit electrons when light shines on them, as long as the light’s frequency is greater than a certain cut-off valuel. If the light’s frequency is lower than this cut-off for the specific metal, electrons will not be ejected no matter how bright the light is, which seems strange since the energy in a light wave is proportional to its intensity.
Many metals will emit electrons when light shines on them, as long as the light’s frequency is greater than a certain cut-off valuel. If the light’s frequency is lower than this cut-off for the specific metal, electrons will not be ejected no matter how bright the light is, which seems strange since the energy in a light wave is proportional to its intensity.
Einstein explained this by theorizing that light consisted of individual photons, each with energy . Since an electron can only be struck by a single photon at once, it will only receive enough energy to escape the surface of the metal if the photon’s frequency is high enough. The minimum energy necessary to eject an electron from the surface of a metal is called its work function . The maximum kinetic energy of electrons ejected by photons of frequency will be
An aluminum can electroscope can provide a useful way of demonstrating the photoelectric effect. The work function of aluminum is roughly 4.15 eV. The electroscope can be given an excess of electrons via the triboelectric effect, and then it becomes easy to see when those electrons have been released from the metal surface of the electroscope by a light source of the appropriate frequency.