Planck Constant Measurement
Objectives
- Measure h/q_e, the ratio of Planck’s Constant and electron charge, using the photoelectric effect
- Measure the work function of a metal using the photoelectric effect.
Resources
- PASCO Photoelectric Effect Apparatus
- Mercury light source
- Photodiode enclosure with variable aperture and color filters
- DC current amplifier
- DC power supply (30V range)
- Voltmeter
Background
A photon of sufficient energy h\nu, where h=6.626\times10^{-34}\mathrm{\ J\cdot s} is Planck’s constant and \nu the frequency of the photon, can give an electron enough energy to eject it from its atom. In metals this is especially apparent, as there are a lot of loosely held electrons.
The minimum energy necessary to eject an electron from the surface of a given metal is its work function W_0. The metal in the photodiode used in this experiment is a cesium-antimiony semiconductor with a work function of W_0 = 1.36 \pm 0.08 \mathrm{eV}.
The work function of a metal can be measured using a setup like that shown schematically below. The electrons ejected by the photoelectric produce a measurable current. Applying a voltage bias from the anode to the cathode of the photodiode produces an electric field that decelerates the ejected electrons. If the voltage is increased until the photoelectric current becomes zero, the maximum kinetic energy of the ejected electrons must be q_eV, which must also be equal to h\nu-W_0, the energy of the incident photon minus the metal’s work function.
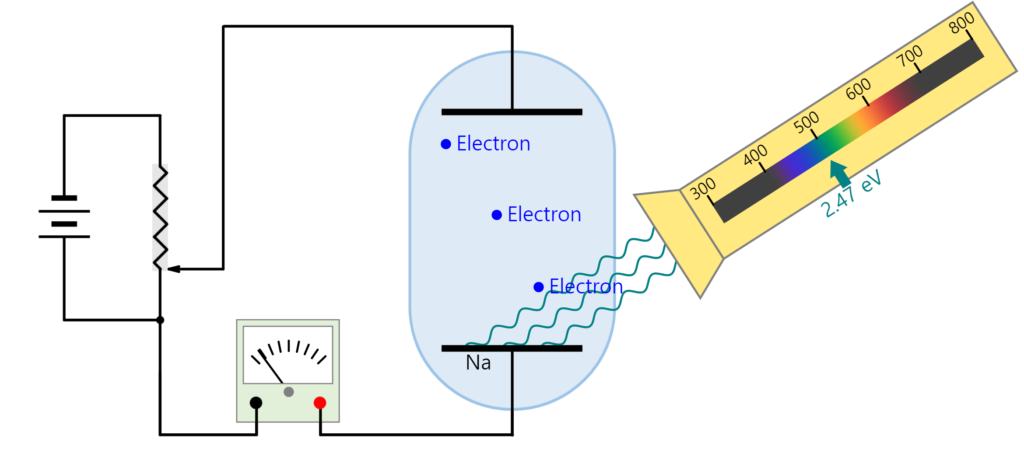 Method
Method
Connect the apparatus as shown in the image above. Turn on the mercury lamp and let it warm up for at least 10 minutes. Rotate the aperture selector on the photodiode enclosure to allow different wavelengths of light from the mercury lamp to hit the photodiode. For each light wavelength, adjust the voltage on the power supply until the photocurrent measured by the amplifier reads zero. According to the discussion above, the relationship between the light frequency \nu and stopping voltage V should be V=\frac{h}{q_e}\nu-\frac{W_0}{q_e}
Analysis Questions
- What values do you obtain for the value of h/q_e and W_0? Do they agree within your uncertainty?
- What is the cutoff frequency of this metal i.e. the minimum frequency needed to eject electrons?
- How would you use this apparatus to show that the ability to eject electrons does not depend on the intensity of the incident light? Explain your method and do the measurement.