Heat Transfer
Tasks
- Melt some ice in water, and measure how much the water’s temperature changes to determine the heat energy released by melting ice (its latent heat of fusion).
- (optional) Determine the specific heat of an unknown metal, and use that to identify it.
Resources
- Calorimeter
- Digital thermometer
- Ice
- Scale
- Stopwatch (www.onlinestopwatch.net)
- Steam Generator (if heating metals)
- Small cylinder of an unknown metal
Background
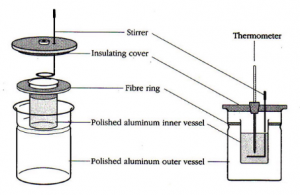
Heat is the amount of energy that transfers from a warmer object to a cooler one. Like energy, it is measured in Joules. A calorimeter is a container that is designed to insulate its contents from the outside environment as much as possible. This is accomplished by an insulator such as styrofoam (or just air in some cases) between the inner and outer vessels shown in the diagram below. The inner cup, its contents, and the metal stirrer are all in thermal contact, so heat can transfer between them but does not exchange with the outside environment (or does so slowly). The stirrer is used to help keep the temperature of the contents uniform, so the temperature reading is accurate.
For many substances, the relationship between the amount of heat energy Q added or lost per unit mass is proportional to the temperature change. This is written as Q=mc\Delta T where m is the mass and c the specific heat for the substance. Some common specific heats are:
- Water: c = 4.184 joules/gram/°C
- Aluminum: c = 0.897 joules/gram/°C
- Copper: c = 0.385 joules/gram/°C
- Iron: c = 0.412 joules/gram/°C
The SI unit of heat is the same as energy – the Joule. Heat can also be measured in calories. One calorie is equal to 4.184 Joules. When a substance changes phase (from liquid to gas, solid to liquid, etc.) some heat energy must be lost or gained in order to effect the change. For example, when ice melts some heat energy must be used to break the intermolecular bonds that give ice its rigid structure. This energy does not go into raising the temperature of the ice. The heat required to melt a mass m of ice is Q_{fusion}=mL where the latent heat of fusion of ice is L = 333.55 joules/gram.
An ice cube placed in lukewarm water will slowly melt as heat from the water transfers to it. As the ice melts, it will convert into an equal mass of water that will continue to be warmed by the surrounding water until the temperatures equalize, and this additional mass of water must also be accounted for.
The total heat change of the system for melting an ice cube in the calorimeter will be Q_{H_2O}+Q_{cup}+Q_{ice}+ Q_{fusion} = 0 Q=mc\Delta T Q_{fusion}=mL
Guideposts
- It is difficult to accurately measure the temperature of the inner metal cup and stirrer directly. However, if you wait a couple minutes before adding ice, they will have time to come to thermal equilibrium with any water in the cup.
- A small ice cube that is sitting out in a room and is just beginning to melt can be assumed to have a uniform temperature of 0°C.